Blog
Pool Water Balancers
October 26, 2021| Posted in Swimming Pools, Pool Balancers| 12414
Explaining the importance of balanced pool water
How does your water supply affect your Spa or Hot Tub?
An explanation on how your water supply can affect the maintenance of your spa or hot tub.
Why do I need to shock my pool?
An explanation on why shocking your pool is important
Hot Tub Starter Kits
A short guide on hot tub starter kits
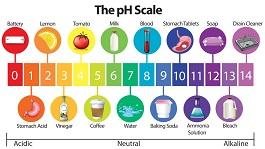
Alkalinity and Ph in pools
February 19, 2021| Posted in Swimming Pools, Pool Balancers| 1121
Understanding the relationship between Alkalinity and Ph in pools
Calcium Hypochlorite Pellets
February 12, 2021| Posted in Swimming Pools, Calcium Hypochlorite| 764
A short description about calcium hypochlorite pellets
Opening your Pool
February 12, 2021| Posted in Swimming Pools, Opening your Pool| 743
A step by step guide on how to open your pool for the summer
First Time Buyers
A summary of what's needed when first buying a pool or spa
Chlorine or Bromine?
February 4, 2021| Posted in Spas| 1038
A comparison between running spas on chlorine or bromine.
Show
per page